The Institute Colloquium: Feedback Control of Signal Transduction by Dynamic Actin Ne
Date
Monday, May 27, 2013 16:30 - 17:30
Speaker
Jack Taunton (University of California, San Francisco)
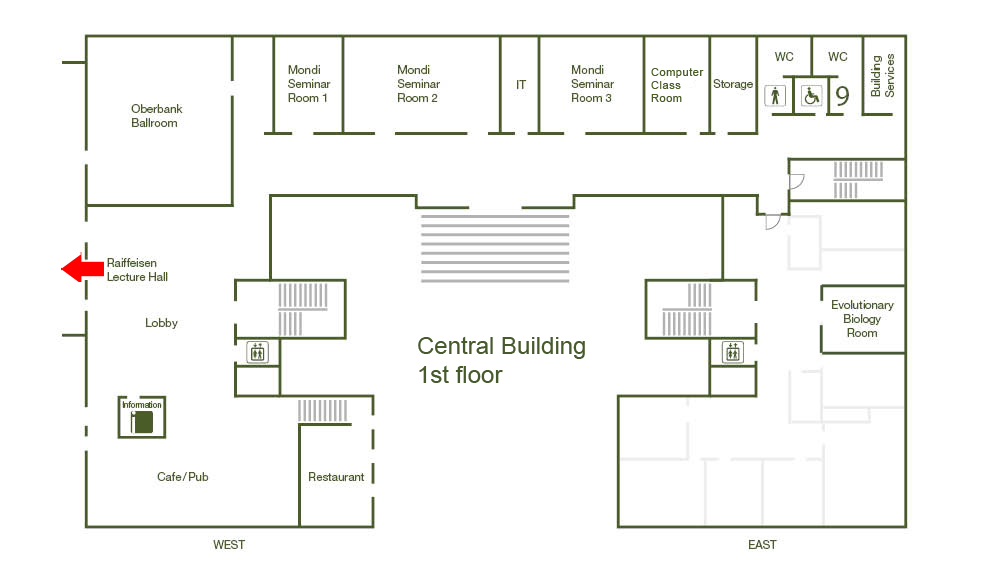
Location
Raiffeisen Lecture Hall, Central Building
Series
Colloquium
Tags
Institute Colloquium
Contact
Actin polymerization drives membrane movement and is spatially regulated by membrane-associated signaling complexes. Rho-family GTPases (such as Rac and Cdc42) bind and activate actin nucleation factors, which localize to discrete zones--- along the membrane corresponding to sites of actin polymerization and membrane protrusions. However, it is unclear how actin networks spatially organize signaling proteins into clusters and how this spatial organization affects signal transduction. By reconstituting a Cdc42-driven signaling cascade from purified proteins and supported lipid bilayers, we have gained mechanistic insights into how actin dynamics exert local feedback control on signal transduction events at the membrane.